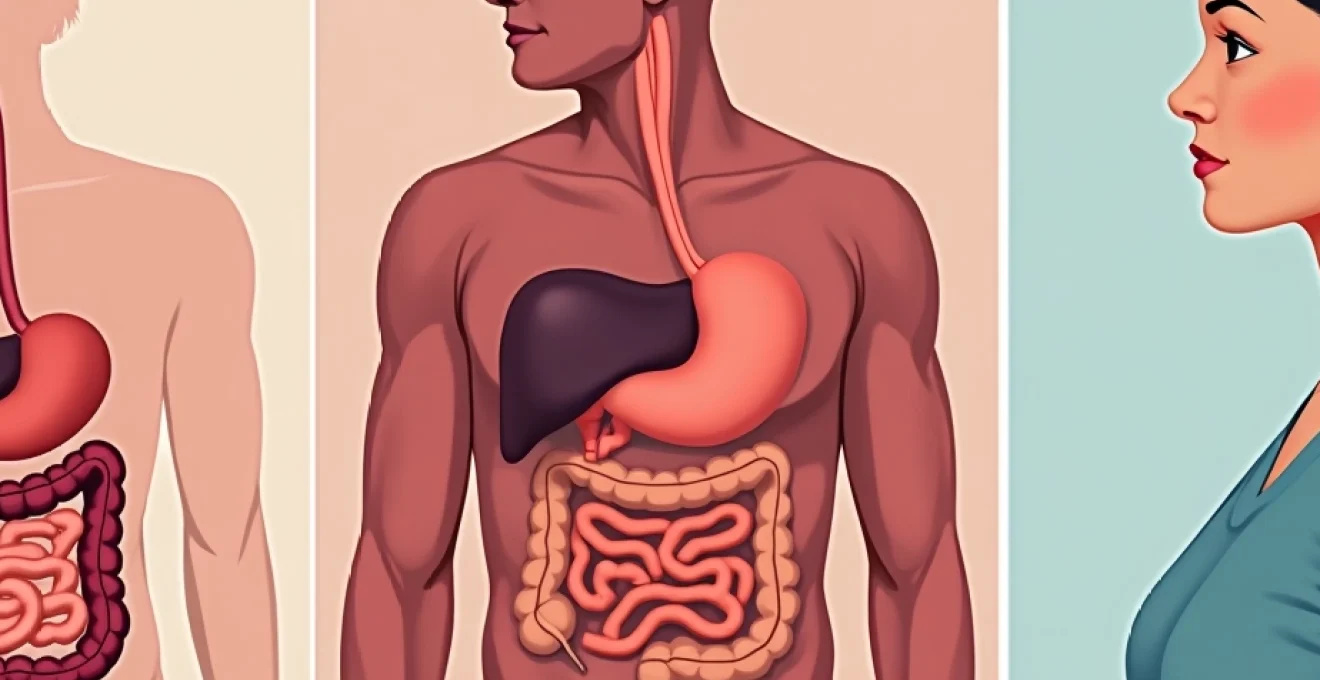
Crohn’s disease affects millions of people worldwide, representing one of the most challenging inflammatory bowel diseases that can significantly impact quality of life. This chronic condition causes inflammation throughout the digestive tract, leading to a complex array of symptoms that can range from mild discomfort to debilitating complications. Understanding the intricate mechanisms behind Crohn’s disease, recognising its diverse clinical presentations, and exploring comprehensive treatment approaches are essential for both patients and healthcare professionals managing this lifelong condition.
The journey with Crohn’s disease often begins with subtle symptoms that can be easily dismissed or attributed to other conditions. However, as the inflammatory process progresses, patients typically experience increasingly severe manifestations that require immediate medical attention and long-term management strategies. Modern advances in diagnostic imaging, targeted therapies, and surgical techniques have revolutionised treatment outcomes, offering hope for sustained remission and improved quality of life.
Inflammatory bowel disease pathophysiology: understanding crohn’s disease mechanisms
The pathophysiology of Crohn’s disease involves a complex interplay between genetic predisposition, environmental triggers, and immune system dysfunction. Unlike other inflammatory conditions, Crohn’s disease demonstrates unique characteristics that distinguish it from similar gastrointestinal disorders. The inflammatory process in Crohn’s disease can affect any part of the digestive tract, from the mouth to the anus, though it most commonly involves the terminal ileum and colon.
Transmural intestinal wall inflammation and granulomatous response
One of the hallmark features of Crohn’s disease is its transmural nature, meaning the inflammation extends through all layers of the intestinal wall. This deep penetration creates a distinctive pathological pattern that can lead to complications such as strictures, fistulas, and abscesses. The inflammatory infiltrate typically consists of lymphocytes, plasma cells, and macrophages, creating a chronic inflammatory environment that perpetuates tissue damage.
The granulomatous response observed in approximately 60% of Crohn’s disease cases represents another distinguishing feature. These microscopic clusters of immune cells, known as epithelioid granulomas, form when the immune system attempts to contain perceived threats. The presence of non-caseating granulomas in biopsy specimens often supports the diagnosis of Crohn’s disease, though their absence doesn’t rule out the condition.
Dysregulated immune system activation in Gut-Associated lymphoid tissue
The gut-associated lymphoid tissue (GALT) plays a crucial role in maintaining intestinal homeostasis and immune surveillance. In Crohn’s disease, this delicate balance becomes disrupted, leading to an inappropriate inflammatory response against normally harmless intestinal bacteria. The dysregulation involves both innate and adaptive immune systems, creating a self-perpetuating cycle of inflammation.
T-helper cells, particularly Th1 and Th17 subsets, become overactivated and produce excessive amounts of pro-inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin-12, and interferon-gamma. This cytokine storm contributes to tissue damage and perpetuates the inflammatory cascade. Understanding these immune mechanisms has led to the development of targeted biologic therapies that specifically inhibit these inflammatory pathways.
Genetic polymorphisms in NOD2 and ATG16L1 susceptibility genes
Genetic research has identified over 200 susceptibility loci associated with Crohn’s disease, with the NOD2 gene being the first and most extensively studied. Mutations in the NOD2 gene occur in approximately 15-20% of Crohn’s disease patients and affect the protein’s ability to recognise bacterial components, leading to impaired immune responses. These genetic variants are particularly common in European populations and contribute to increased disease susceptibility.
The ATG16L1 gene, involved in autophagy pathways, represents another significant genetic factor. Autophagy is a cellular process that helps eliminate damaged organelles and pathogens, and dysfunction in this pathway can contribute to chronic inflammation. Other important susceptibility genes include IL23R, IRGM, and CARD9, each contributing to different aspects of immune function and inflammatory responses.
Mycobacterium avium subspecies paratuberculosis environmental triggers
Environmental factors play a crucial role in triggering Crohn’s disease in genetically susceptible individuals. Mycobacterium avium subspecies paratuberculosis (MAP) has been identified as a potential environmental trigger, as this organism causes Johne’s disease in ruminants, which shares similarities with Crohn’s disease. Some studies have detected MAP DNA in intestinal tissues of Crohn’s disease patients, though its causative role remains controversial.
Other environmental factors that may contribute to disease development include dietary factors, smoking, non-steroidal anti-inflammatory drugs (NSAIDs), and infections. The hygiene hypothesis suggests that reduced early-life exposure to microorganisms may contribute to the development of inflammatory bowel diseases, as seen by higher incidence rates in developed countries and urban environments.
Intestinal microbiome dysbiosis and reduced bacterial diversity
The intestinal microbiome in Crohn’s disease patients typically shows reduced diversity and altered composition compared to healthy individuals. This dysbiosis is characterised by decreased beneficial bacteria such as Faecalibacterium prausnitzii and increased potentially harmful species like adherent-invasive Escherichia coli (AIEC). The altered microbiome contributes to increased intestinal permeability and sustained inflammatory responses.
Research demonstrates that Crohn’s disease patients often have a 25-50% reduction in microbial diversity compared to healthy controls, with significant implications for immune function and barrier integrity.
Clinical manifestations and diagnostic criteria for crohn’s disease
The clinical presentation of Crohn’s disease varies significantly depending on the location, extent, and severity of inflammation. Symptoms can develop gradually over months or appear suddenly, often mimicking other gastrointestinal conditions. The unpredictable nature of symptom onset and progression makes early diagnosis challenging, with many patients experiencing delays in appropriate treatment initiation.
Abdominal pain patterns in terminal ileum and right lower quadrant
Abdominal pain represents one of the most common and troublesome symptoms experienced by Crohn’s disease patients. The pain typically localises to the right lower quadrant when the terminal ileum is involved, often mimicking appendicitis in acute presentations. The pain character can vary from cramping and colicky to constant and severe, frequently worsening after meals due to increased intestinal motility.
The intensity and pattern of abdominal pain often correlate with disease activity and location. Patients with stricturing disease may experience severe cramping pain associated with partial bowel obstruction, while those with penetrating disease might develop persistent, deep-seated pain. Understanding these pain patterns helps clinicians localise disease involvement and guide appropriate diagnostic investigations.
Chronic diarrhoea with blood and mucus characteristics
Chronic diarrhoea affects approximately 80-90% of Crohn’s disease patients and can significantly impact quality of life. The diarrhoea in Crohn’s disease may be watery, bloody, or contain mucus, depending on the location and extent of inflammation. Small bowel involvement typically produces watery diarrhoea due to malabsorption, while colonic involvement more commonly causes bloody stools with mucus.
The frequency of bowel movements can range from 3-4 times daily in mild disease to more than 20 times daily during severe flares. Nocturnal diarrhoea, urgency, and faecal incontinence are particularly distressing symptoms that can lead to social isolation and psychological distress. The chronic nature of diarrhoea in Crohn’s disease also contributes to dehydration, electrolyte imbalances, and nutritional deficiencies.
Extra-intestinal complications: arthritis, uveitis and erythema nodosum
Extra-intestinal manifestations occur in approximately 25-40% of Crohn’s disease patients and can sometimes precede gastrointestinal symptoms. Arthritis represents the most common extra-intestinal complication, affecting both peripheral joints and the axial skeleton. Peripheral arthropathy typically involves large joints and correlates with intestinal disease activity, while ankylosing spondylitis follows an independent course.
Ocular manifestations, including episcleritis and uveitis, can cause significant morbidity if left untreated. Uveitis, in particular, can lead to permanent vision loss and requires urgent ophthalmological evaluation. Skin manifestations such as erythema nodosum and pyoderma gangrenosum occur in 10-15% of patients and often parallel intestinal disease activity. These dermatological complications can be both physically and emotionally challenging for patients.
Weight loss and malnutrition from malabsorption syndrome
Malnutrition and weight loss are common complications of Crohn’s disease, affecting up to 75% of patients at some point during their disease course. The malnutrition results from multiple factors including reduced oral intake due to pain and nausea, malabsorption secondary to intestinal inflammation, increased metabolic demands from chronic inflammation, and nutrient losses from diarrhoea.
Specific nutrient deficiencies commonly observed include iron deficiency anaemia, vitamin B12 deficiency (particularly with ileal involvement), folate deficiency, and fat-soluble vitamins (A, D, E, K). Protein-energy malnutrition can be particularly severe in hospitalised patients and requires comprehensive nutritional assessment and intervention. Early recognition and treatment of nutritional deficiencies are essential for optimising treatment outcomes and preventing long-term complications.
Advanced diagnostic imaging and endoscopic techniques
Accurate diagnosis of Crohn’s disease requires a combination of clinical assessment, laboratory investigations, endoscopic evaluation, and cross-sectional imaging. No single test can definitively diagnose Crohn’s disease; instead, clinicians rely on a constellation of findings to establish the diagnosis. The diagnostic approach has evolved significantly with technological advances, allowing for more precise disease characterisation and monitoring.
Colonoscopy with ileoscopy for mucosal assessment and biopsy collection
Colonoscopy with ileoscopy remains the gold standard for evaluating colonic and terminal ileal involvement in Crohn’s disease. This procedure allows direct visualisation of mucosal changes, including ulceration, cobblestone appearance, and skip lesions characteristic of Crohn’s disease. The ability to obtain tissue biopsies for histopathological examination provides crucial diagnostic information, particularly for identifying granulomas and ruling out malignancy.
Endoscopic findings in Crohn’s disease include aphthous ulcers in early disease, linear ulcers, cobblestone mucosa, and strictures in advanced cases. The patchy distribution of inflammation, known as skip lesions, helps differentiate Crohn’s disease from ulcerative colitis. Modern high-definition endoscopes and advanced imaging techniques such as narrow-band imaging enhance the detection of subtle mucosal changes and improve diagnostic accuracy.
Magnetic resonance enterography for small bowel evaluation
Magnetic resonance enterography (MRE) has emerged as the preferred cross-sectional imaging technique for evaluating small bowel Crohn’s disease. This non-invasive technique provides excellent soft tissue contrast and can detect inflammation, strictures, and complications such as abscesses and fistulas without exposing patients to ionising radiation. MRE is particularly valuable for monitoring disease progression and treatment response.
The technique involves oral contrast administration to distend the small bowel, followed by intravenous gadolinium to enhance inflammatory changes. Key MRE findings in Crohn’s disease include bowel wall thickening, increased T2 signal intensity, restricted diffusion, and mesenteric changes. MRE sensitivity for detecting active small bowel inflammation exceeds 90% , making it an invaluable tool for diagnosis and monitoring.
Computed tomography enteroclysis in acute complications
Computed tomography enteroclysis (CTE) plays a crucial role in evaluating acute complications of Crohn’s disease, particularly in emergency settings. While MRE is preferred for routine monitoring due to radiation concerns, CT provides rapid assessment of patients presenting with acute abdominal pain, suspected perforation, or bowel obstruction. The speed and availability of CT make it ideal for urgent clinical scenarios.
CTE findings in Crohn’s disease include the “comb sign” (engorged mesenteric vessels), target sign (layered bowel wall enhancement), and complications such as abscesses, free perforation, or high-grade obstruction. The technique is particularly useful for surgical planning and can help differentiate inflammatory strictures from fibrotic ones based on enhancement patterns.
Capsule endoscopy for inaccessible small bowel segments
Capsule endoscopy provides direct visualisation of the small bowel mucosa in areas inaccessible to conventional endoscopy. This technique is particularly valuable for evaluating suspected small bowel Crohn’s disease when other imaging modalities are inconclusive. The capsule captures thousands of images as it traverses the gastrointestinal tract, allowing for detailed mucosal assessment.
Capsule endoscopy demonstrates superior sensitivity for detecting small bowel inflammation compared to conventional radiological techniques. However, the risk of capsule retention in patients with suspected strictures requires careful patient selection and often necessitates prior patency capsule assessment. The technique has transformed the evaluation of obscure gastrointestinal bleeding and small bowel inflammation in appropriate candidates.
Studies indicate that capsule endoscopy can detect small bowel lesions in up to 70% of patients with suspected Crohn’s disease and normal conventional investigations, significantly impacting diagnostic yield.
Pharmacological treatment algorithms and biologic therapies
The treatment landscape for Crohn’s disease has undergone revolutionary changes with the introduction of biologic therapies and targeted small molecules. Modern treatment algorithms emphasise early aggressive therapy to achieve deep remission and prevent long-term complications. The concept of “treat-to-target” has gained prominence, focusing on objective measures of inflammation rather than symptom control alone.
Treatment selection depends on multiple factors including disease severity, location, presence of complications, and patient preferences. The traditional step-up approach, beginning with conventional therapies and escalating to biologics, is increasingly being challenged by top-down strategies that introduce effective therapies early in the disease course. Personalised medicine approaches are emerging, utilising genetic markers and biomarkers to predict treatment response.
Conventional therapies include corticosteroids for acute flares, immunomodulators such as azathioprine and methotrexate for maintenance therapy, and 5-aminosalicylates for mild disease. However, the limitations of these agents, including significant side effects and suboptimal efficacy, have led to the development of more targeted approaches. The introduction of anti-TNF agents marked a paradigm shift in Crohn’s disease management, followed by additional biologic classes targeting different inflammatory pathways.
Anti-TNF agents, including infliximab, adalimumab, and certolizumab pegol, have demonstrated efficacy in both induction and maintenance of remission. These agents block tumour necrosis factor-alpha, a key pro-inflammatory cytokine in Crohn’s disease pathogenesis. Anti-integrin therapies like vedolizumab offer gut-selective immunosuppression by blocking lymphocyte trafficking to the gastrointestinal tract. Anti-interleukin-12/23 agents such as ustekinumab provide another therapeutic option for refractory disease.
The newest additions to the therapeutic armamentarium include JAK inhibitors like upadacitinib, which target intracellular signalling pathways involved in inflammation. These oral agents offer convenience advantages over injectable biologics while maintaining significant efficacy. Biosimilar agents have increased accessibility to biologic therapies while reducing healthcare costs, making these advanced treatments available to more patients worldwide.
Surgical interventions and post-operative management strategies
Surgery plays a crucial role in Crohn’s disease management, with approximately 70-80% of patients requiring at least one surgical procedure during their lifetime. Unlike ulcerative colitis, surgery is not curative for Crohn’s disease due to its potential to affect any part of the gastrointestinal tract. However, surgical intervention can provide significant symptom relief and improve quality of life when medical therapy fails or complications develop.
Common indications for surgery include stricturing disease causing bow
el obstruction, perforation, refractory bleeding, and failure of medical therapy. The timing of surgical intervention requires careful consideration, balancing the risks of continued medical therapy against surgical morbidity. Early surgical consultation is recommended for patients with complications, as delayed intervention may increase operative risks and compromise outcomes.
Laparoscopic approaches have become the standard of care for most Crohn’s disease operations, offering reduced postoperative pain, shorter hospital stays, and improved cosmetic outcomes. Ileocolonic resection remains the most common surgical procedure, typically performed for terminal ileal disease with or without cecal involvement. Strictureplasty techniques allow preservation of bowel length in patients with multiple strictures or short gut syndrome, reducing the risk of short bowel syndrome.
Post-operative management focuses on preventing recurrence and optimising long-term outcomes. Endoscopic recurrence occurs in up to 90% of patients within one year of surgery, while clinical recurrence affects 20-30% of patients within five years. Post-operative prophylaxis with immunomodulators or biologic agents significantly reduces recurrence rates, particularly in high-risk patients. Risk stratification based on smoking status, perforating disease, and prior resections guides prophylactic therapy decisions.
The concept of enhanced recovery after surgery (ERAS) protocols has been successfully implemented in Crohn’s disease surgery, reducing complications and hospital length of stay. These protocols encompass pre-operative optimisation, minimally invasive techniques, and structured post-operative care pathways. Nutritional optimisation before surgery is particularly important, as malnutrition increases operative risks and delays healing.
Research demonstrates that patients receiving post-operative prophylaxis with anti-TNF therapy have a 60-70% reduction in clinical recurrence compared to those receiving no prophylaxis, highlighting the importance of preventive strategies.
Nutritional management and lifestyle modifications for disease control
Nutritional management represents a cornerstone of comprehensive Crohn’s disease care, addressing both the consequences of chronic inflammation and the potential therapeutic benefits of dietary interventions. Malnutrition affects up to 85% of hospitalised Crohn’s disease patients and can significantly impact treatment outcomes, wound healing, and overall prognosis. A multidisciplinary approach involving gastroenterologists, dietitians, and specialised nutrition teams ensures optimal nutritional care.
Exclusive enteral nutrition protocols for pediatric populations
Exclusive enteral nutrition (EEN) has emerged as a highly effective therapy, particularly in pediatric Crohn’s disease, where it serves as first-line induction therapy. This approach involves replacing normal diet with nutritionally complete liquid formulas for 6-8 weeks, achieving remission rates comparable to corticosteroids without the associated side effects. EEN promotes mucosal healing, reduces inflammatory markers, and supports normal growth and development in children.
The mechanism of EEN effectiveness involves bowel rest, alteration of intestinal microbiota, and provision of optimal nutrition for healing. Different formula types, including elemental, semi-elemental, and polymeric formulations, show similar efficacy rates. Patient adherence remains the primary challenge with EEN therapy, requiring extensive patient and family education, psychological support, and creative strategies to maintain compliance throughout the treatment period.
Mediterranean diet patterns and anti-inflammatory food choices
Emerging evidence suggests that specific dietary patterns may influence Crohn’s disease activity and progression. The Mediterranean diet, characterised by high consumption of fruits, vegetables, whole grains, legumes, and olive oil, demonstrates anti-inflammatory properties that may benefit Crohn’s disease patients. This dietary pattern is rich in omega-3 fatty acids, antioxidants, and fibre, which collectively support intestinal barrier function and reduce systemic inflammation.
Specific foods showing potential benefits include fatty fish rich in omega-3 fatty acids, fermented dairy products containing beneficial probiotics, and polyphenol-rich foods such as berries and green tea. Conversely, ultra-processed foods, excessive red meat consumption, and foods high in emulsifiers may exacerbate inflammation. The Crohn’s Disease Exclusion Diet (CDED) represents a structured approach to dietary therapy, showing promising results in clinical trials when combined with partial enteral nutrition.
Micronutrient supplementation and deficiency prevention
Systematic assessment and correction of micronutrient deficiencies form essential components of Crohn’s disease management. Iron deficiency anaemia affects 60-80% of patients and requires careful evaluation to distinguish between iron deficiency and anaemia of chronic disease. Intravenous iron therapy often proves more effective than oral supplementation in active disease, providing faster correction with better tolerance.
Vitamin B12 deficiency commonly occurs with ileal involvement, requiring parenteral replacement in severe deficiencies. Vitamin D deficiency affects over 75% of Crohn’s disease patients and requires aggressive replacement given its role in immune function and bone health. Folate deficiency may result from malabsorption or methotrexate therapy, while fat-soluble vitamin deficiencies (A, D, E, K) occur with significant malabsorption or bile acid deficiency.
Smoking cessation and substance use counselling
Smoking represents the most important modifiable risk factor in Crohn’s disease, doubling the risk of disease development and significantly worsening prognosis. Current smokers experience more frequent flares, increased hospitalisation rates, greater need for immunosuppressive therapy, and higher surgical rates compared to non-smokers. Smoking cessation should be prioritised in all Crohn’s disease patients who smoke, with intensive counselling and pharmacological support offered.
The benefits of smoking cessation become apparent within months of quitting, with reduced flare rates and improved response to medical therapy. Nicotine replacement therapy, bupropion, and varenicline can be safely used in Crohn’s disease patients, though careful monitoring is required. Cannabis use, while sometimes employed for symptom management, lacks robust evidence for therapeutic benefit and may complicate treatment assessment.
Stress management and psychological intervention strategies
Chronic stress significantly impacts Crohn’s disease course through neuro-immune pathways that promote inflammation and alter gut barrier function. The bidirectional gut-brain axis means that intestinal inflammation can affect mood and cognition, while psychological stress can exacerbate gastrointestinal symptoms. Comprehensive management must address both physical and psychological aspects of the disease.
Evidence-based psychological interventions include cognitive-behavioural therapy, mindfulness-based stress reduction, and gut-directed hypnotherapy. These approaches help patients develop coping strategies, reduce anxiety and depression, and may improve disease outcomes. Regular physical activity, when tolerated, provides additional benefits through stress reduction, improved immune function, and enhanced quality of life. Sleep hygiene and adequate rest are equally important, as sleep disturbances can worsen inflammation and impair treatment response.
Studies show that patients participating in structured stress management programmes experience 40% fewer flares and significantly improved quality of life scores compared to those receiving standard medical care alone.
The integration of nutritional therapy, lifestyle modifications, and psychological support with medical and surgical treatments represents the optimal approach to Crohn’s disease management. This comprehensive strategy addresses the multiple factors contributing to disease activity while supporting overall health and wellbeing. Regular monitoring and adjustment of these interventions ensure continued effectiveness and help patients achieve the best possible outcomes in their journey with Crohn’s disease.